The fate of carbon monoxide in the atmosphere and oceans
The carbon monoxide concentration in the atmosphere is increasing by 2-3 ppm annually. This adds to the absorption of CO2 in the atmosphere. To put this in perspective we can examine the absorption of gases in the atmosphere both in the ultrviolet-visible region (for incident sunlight) and in the infrared-microwave (for light emitted by the earth). This is shown in the figure below:
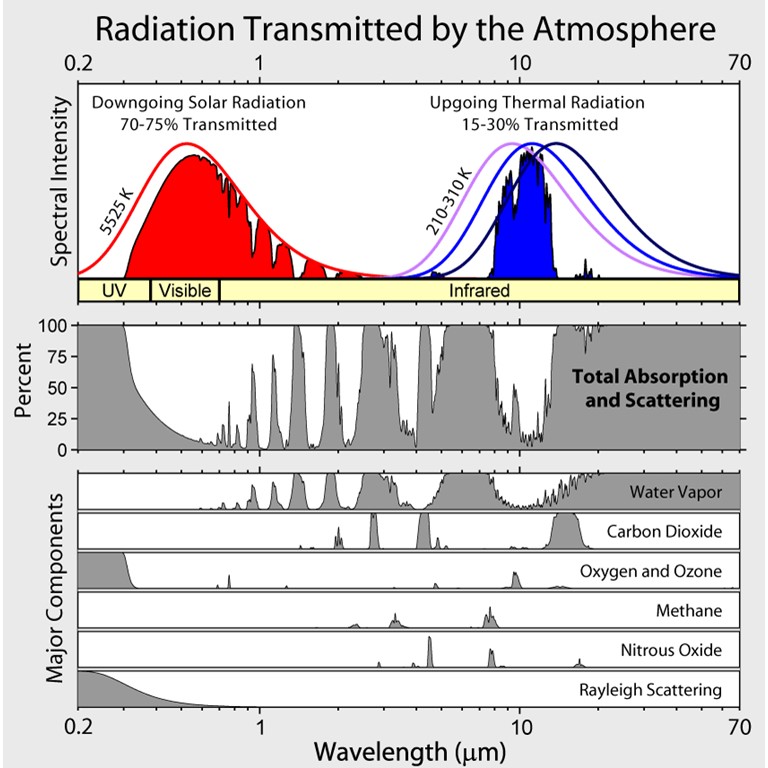
The thermal radiation of the sun is peaked ca. 550 nm while that of the earth is at 11 microns (11,000 nm). This difference is inversely proportional to their temperatures according to the Wien displacement law.
λmax T = c2
where c2 is the second radiation constant. In practice if the wavelength is in units of nm and temperature in K the value of the constant is 2.88 x 106 nm-K.
The carbon cycle
To fully understand how carbon dioxide builds up in the atmosphere over time we need to consider the full range of process whereby carbon is taken up by the oceans, deposited as calcaium carbonate (limestone), replaced by silicon in calcium silicate (releasing CO2 and taken up by plants and photosynthetic organisms.
It is remarkable how many of these processes do not reach equilibrium. Of course, uptake of carbon dioxide by plants and photosynthetic bacteria is a steady state process that traps the carbon only for as long as the plants/bacteria are alive. In addition, CO2 released into the atmosphere is taken up by the oceans (see below). In the oceans carbon dioxide forms carbonate, which can precipitate as calcium carbonate. Although the thermodynamic driving force for formation of calcium carbonate is large the precipitation is frustrated in most of the ocean since CaCO3 redissolves at a depth of about 4500 meters due to the pressure at that depth.
The greenhouse effect
Carbon dioxide absorbs infrared radiation mainly due to its bending mode at 15 microns. The bending mode is quite complex due to numerous rotational subbands, Fermi resonance and numerous isotopomers of CO2. The HITRAN database reports nearly 1,000,000 transitions from 10-20 microns due to CO2. The absorption of heat (infrared radiation) emitted by the earth results in warming of the atmosphere. While warming keeps the earth temperate and permits life to exist, too much warming results in melting of the polar ice caps, changes in weather patterns due to changes in air flows in response to heating of the oceans. In general, the stability of the climate is affected.
The fate of carbon dioxide in the oceans
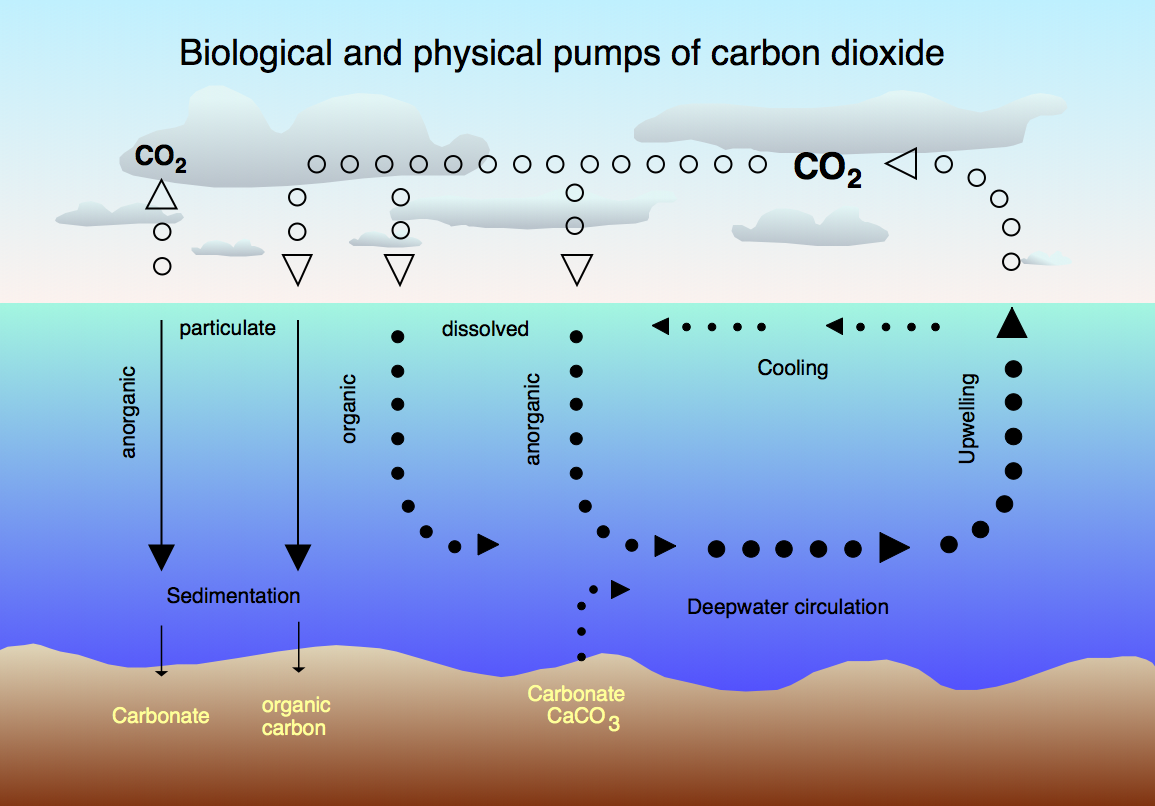
Figure from Hannes Grobe, Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, Germany
The figure showing biological and physical pumps of CO2 gives more detail concerning the processes the determine the trapping and release of CO2 by the ocean than we need for the big picture calculation of CO2 uptake. The fundamental equilibrium is the Henry's law equilibrium between CO2 (g) and CO2 (aq). Of course, in the ocean there are further equilibria including the reaction of CO2 with H2O to make carbonic acid. Carbonic has two acidic equilibria. Formation of carbonate can lead to precipitation in the presence of ions such as Ca2+ or Mg2+. This section also discusses the physical processes that prevent complete precipitation of CaCO3 in the deep ocean. The co-named Carbon Compensation Depth (CCD) is the depth at which calcium carbonate redissolves due to the hydrostatic pressure. This depth is ca. 5000 meters so the effect is only important in the deep ocean. But, most of the ocean is deep ocean so this kinetic trap is extremely important for the actual ocean chemistry.
Ocean acidification
As the concentration of CO2 in the atmosphere increases (measured in ppm) the concentration in the ocean will increase in parallel. In the previous section we have shown that approximately 50% of the CO2 pumped into the atmosphere is absorbed by the ocean. Dissolved CO2 is an acid that has two dissociation equilibria releasing [H+] and thereby decreasing the pH. The ocean is buffered by the presence of two acidic equilibria and this buffering effect tends to mitigate the acidification. Nonetheless, the pH of the ocean has fallen from pH ~ 8.2 at the dawn of the industrial revoltuion to less than pH < 8.1 today. At current rates of CO2 emission the ocean pH will fall below pH ~ 7.8 by 2100. This is dangerously close to a tipping point where photosynthetic organisms in the ocean can no longer survive since their shells will dissolve.
|