CO2 as a greenhouse
gas
There has been a
growing awareness of the problem of CO2 emissions by human
activity. CO2 is an important
greenhouse gas because of the infrared absorption by its bending mode. The bending mode at 667 cm-1 is
sufficiently intense that nearly all of the earth’s emission in the region of 15 micron wavelength is absorbed. This may seem like a fairly
small part of the total spectrum of the earth’s thermal emission, which
is peaked near 11 microns. Yet, infrared
absorption byCO2 in this portion of the spectrum has important
consequences because of feedback loops that lead to greater vaporization of
water. Water is also an important
natural greenhouse gas due to its rotational transitions. As glaciers melt and
temperatures rise there is a greater average vapor pressure. We can understand this from the Clausius-Clapyeron equation, which gives the dependence of
the water vapor pressure on temperature.
From these considerations we see that
spectroscopy and thermodynamics are both important for an understanding of the
role played by CO2 as a greenhouse gas.
We have seen in Chapter
4 that CO2 has four normal modes of vibration. The asymmetric stretch and bending modes are
infrared active. Although CO2
lacks a permanent dipole moment these modes distort CO2 away from
its symmetric structure. The oscillation
creates a time-dependent (and position-dependent) dipole moment. Since the difference dipole moment,![]() , is not zero, the
vibrations have infrared intensity according to the transition moment (Eqn.
4.4.3),
, is not zero, the
vibrations have infrared intensity according to the transition moment (Eqn.
4.4.3),
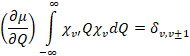
The absorption of radiation occurs
at 667 cm-1 and 2349 cm-1 for the bending and asymmetric
stretching vibrational modes, respectively (see Figure 4.5). This corresponds to wavelengths of 14.9 m and 4.3 m,
respectively. The relative importance of
these modes depends on their position relative to the Earth’s blackbody
emission. The peak of the Earth’s
blackbody emission is given by the Wien displacement law (Eqn. 1.3.1), which
can be written as,
![]()
Thus, the peak of the Eath’s emission is approximately intermediate between the
wavelengths of the two vibrational modes.
The relative importance of the modes is determined by their intensity
(i.e. the magnitude of ![]() . It turns out that the bending mode is the
most important for absorption of the Earth’s radiant heat. The detailed calculation of the effect must
take into account the change in density of the atmosphere, and the fact that
infrared radiation can lead to both absorption and stimulated emission of the
infrared light. This calculation is
beyond the scope of this Chapter.
However, the detailed analysis of the absorption by the CO2
bending mode proves that this mode alone will contribute to heat capture by the
atmosphere proportional to the CO2 partial pressure.
. It turns out that the bending mode is the
most important for absorption of the Earth’s radiant heat. The detailed calculation of the effect must
take into account the change in density of the atmosphere, and the fact that
infrared radiation can lead to both absorption and stimulated emission of the
infrared light. This calculation is
beyond the scope of this Chapter.
However, the detailed analysis of the absorption by the CO2
bending mode proves that this mode alone will contribute to heat capture by the
atmosphere proportional to the CO2 partial pressure.
The
consequences of the heating of the atmosphere produced by increased partial
pressure of CO2 include melting of snow packs and glaciers, and
increased temperature that leads to high water vapor pressure. A small increase in water vapor pressure can
have a large impact on absorption of microwave radiation by the water pure
rotational spectra. Since water has a
large dipole moment along two of its moment of inertia axes, the rotational
absorption gives rise to intense absorption of microwave radiation. These are investigated
further in the Exercises.