The use of horseradish peroxidase as an enzyme for the degradation of environmental pollutants is widespread. The enzyme can be expressed in bacteria and harvested. It is available commercially as a lyophilized powder. It can be activated by addition of H2O2. In its active form it can oxidize a substrate by two electrons. The normal catalytic cycle involves two one-electron oxidations.
Peroxidase activation as a bioremediation enzyme / pdf
Poulos-Kraut Mechanism for activation by H2O2 / pdf
The study of HRP kinetics in the laboratory is actually quite simple. You make a solution with a fixed concentration of HRP and varying concentrations of substrate, e.g. a phenol or other molecule that can be oxidized. The solution is placed in a cuvette in the UV-vis spectrometer in the lab. The spectrometer is set up to collect time-resolved spectra at a rate of about 1 spectrum per second. Then you add H2O2 to initiate the reaction and record the spectra as a function of time.
Protocol for peroxidase kinetic measurements using H2O2
- Time-resolved UV-vis spectra are obtained as a function of concentration of the substrate to permit analysis by Michaelis-Menten kinetics.
Modified Michaelis-Menten kinetic scheme / pdf
- The enzyme kinetic parameters can be studied as a function of temperature, pH, solvent, or different substrate. You may also compare HRP to other peroxidases.
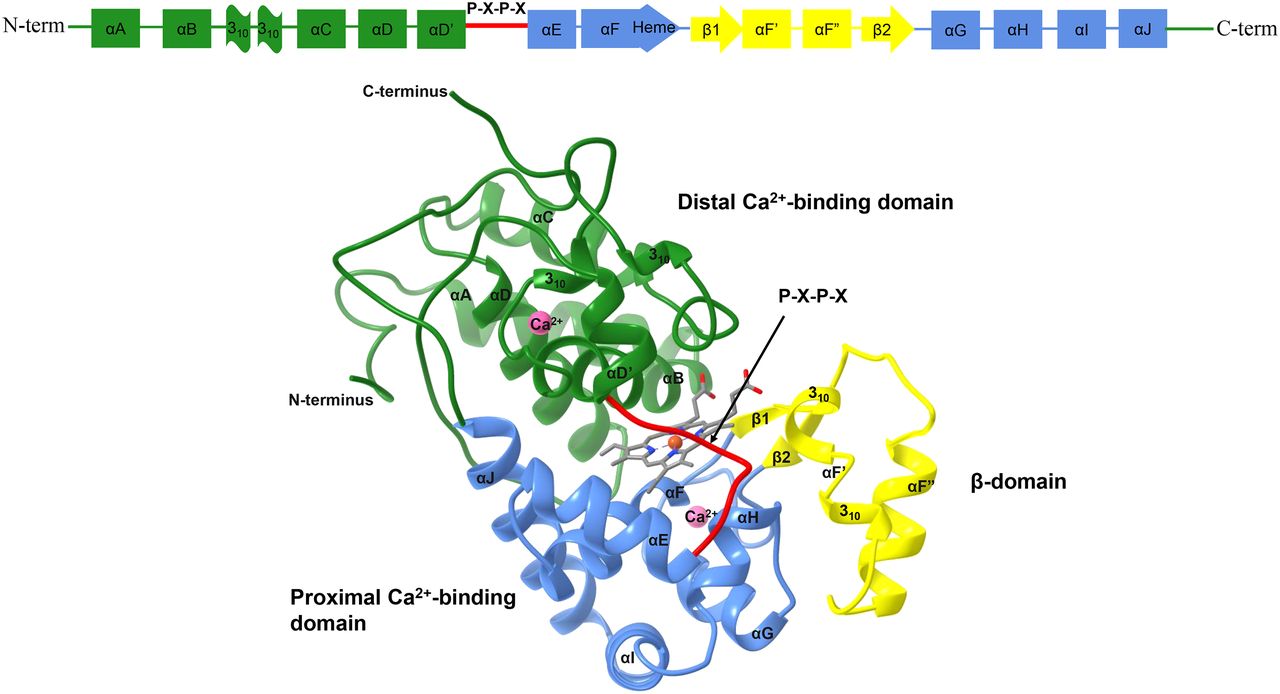
- The research in the Franzen/Ghiladi research laboratories at NCSU focus on studies of Dehaloperoxidase. One option for a research project is to study this enzyme system, which is one of the first examples of a true multi-functional enzyme.
DHP as a multifunctional enzyme / pdf
- The active site for O2 binding in hemoglobin and myoglobin is the heme iron. The heme iron is also the active site in heme enzymes, such as peroxidases, peroxygenases and oxidases. The link below shows the importance of oxidation state and the binding of O2, CO and H2O2.
Binding to ferric and ferrous forms of heme / pdf
- Peroxidase enzymes oxidize substrates on their surface by one-electron oxidation processes. Subsequently, the radicals react to form products. The disproportionation reaction that leads to product formation (and reactant formation) are shown in the link below.
Radical disproportionation in peroxidase catalysis / pdf
|