| |
| Learning about the atmosphere using FTIR? |
| |
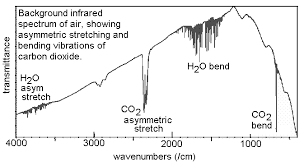
Several atmospheric gases have infrared signatures. By definition, greenhouse gases absorb infrared radiation. These gases form an important aspect of study of the role played by gases in the atmosphere.
This project consists of several options to study CO2 or other gases using FTIR to understand the complex rovibrational spectra and phenomena such as adsorption or interactions of the gases with each other.
- Once you have obtained FTIR spectroscopic data of the carbonyl group of either dimethyl formamide (DMF) or N-methyl acetamide (NNACET) you may compare the calculated frequencies in the DFT calculation files on the right hand column with the experimental data.
- Keep in mind that it is the comparison of the frequencies that is most important. While the actual frequency the carbonyl of DMF may differ from the experimental frequency, the shift in frequency during hydrogen bonding is going to be muc closer to the experimental frequency shift (usually).
- The files on the right are structure files using PDB (Protein Data Bank) format and Density Functional Theory (DFT) output files. The energies are given in several different units and you may use the units most familiar to you.
- The vibrational mode frequencies are given in wavenumbers (cm-1) and are directly comparable to the measured frequencies.
| |
|
| Project information |
 |
| |
References and data |
 |
|
|
|
 |
|
|