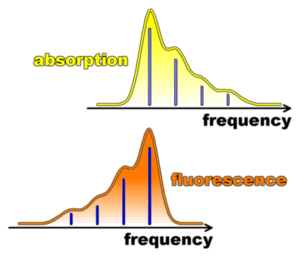
Fluoresence occurs only after an excited state relaxation to a new equilibrium position. Then the molecule can return to the ground state by a vertical transition`. The Franck-Condon factors for the downward transition are the same as those for the upward transition. It is the excited state displacement that determines the nuclear overlap factors and it does not matter whether light is being absorbed or emiitted. This fact gives rise to the mirror image relationship between absorption and emission (fluorescence) spectra. The 0-0' is the same for both absorption and emission. However, the other lines (e.g. 0 - 1', 0 - 2', etc.) are spaced to higher energy for absorption and to lower energy (e.g. 0' - 1, 0' - 2, etc.) for the emission. This is exaplained in greater detail under "mirror image relationship" on the right.
Fluorescence quantum yield
A key point for the emission of light is to determine whether the emission yield is sufficiently high that a fluorecent molecule has application as a molecule probe. Appications of fluorescence have become important in cell imaging, protein orientation, binding and structural changes, materials science and many other specific areas of research. In order to consider applications of fluorescence we have started by studying its line shape as determined by the Franck-Condon factors. However, precisely as is the case for absorption, the square of the transition dipole moment determines the absorption and emission intensity. The Einstein relations show that there is a fixed relationship between the probability of spontaneous emission and the probability for absorption. Incidentally, Einstein also showed that there probability for stimulated emission is equal to that for absorption, which helps to define the characteristics of a laser.
Applications of fluorescence spectroscopy
- Fluorescence quenching
- Fluorescence anisotropy
- Fluorescence resonance energy transfer (FRET)
- Fluorescence recovery after photobleaching (FRAP)
- Fluorescence correlation spectroscopy (FCS)
|