General example of a derivation of the free energy of reaction from the
chemical potential
This example can be extended to a
general case, using an idealized reaction:
aA +
bB ßà
yY + zZ
(1)
The equilibrium constant for this gas
phase reaction is,
![]()
(2)
We will derive this form of the
equilibrium constant in the following. At constant T and P we will write the
total Gibbs energy as
![]()
(3)
In the general case we can write
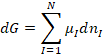
(4)
where we have used capital I to
mean component I. In order to
consolidate the changes in moles we note that we can write all of the molar
changes dni in terms of an overall reaction coordinate dn,
![]()
for products, and
![]()
for reactants. The products have a positive sign since they
are typically being formed and the reactants have a minus sign since they are
being consumed. Thus, for the
hypothetical reaction above we have
![]()
(6)
In Eqn. 8.6.21 the dn represents an
overall reaction progress variable. It
is multiplied by the stoichiometric coefficient of each reactant and the sign
is positive for products and negative for reactants. We have converted the
change in each individual reactant or product into a global variable that
measures how far the reaction has progressed.
We do not yet know how far the reaction will progress. Equilibrium may mean that there is a balance
of reactants and products and dn may be either close to zero (mostly reactants)
or close to 1 (mostly products), by the time the reaction is complete. We now define DrxnG:
![]()
(7)
This definition is unique to
chemistry and lacks a formal justification in the field of mathematics. To
equate a partial derivative quantity with a macroscopic change is not formally
defined. Yet, this step has been used to define the free energy change for more
than 100 years. The importance of Eqn.
8.6.22 is when we realize that it permits us to use the chemical potentials of
individual components to define the collective free energy of a chemical
process. We apply the chemical potential for component I:
![]()
(8)
We can write the Gibbs energy as:
![]()
(9)
and use the chemical potentials for
reactants:
![]()
![]()
and products:
![]()
![]()
(10)
which can be substituted into Eqn.
9,
![]()
![]()
(11)
to obtain
![]()
(12)
where
![]()
(13)
and
![]()
(14)
We note that this gives use the
formal definition of the standard free energy of reaction, ![]() ,
in terms of the chemical potential of the reactants and product. The reaction quotient,
,
in terms of the chemical potential of the reactants and product. The reaction quotient, ![]() ,
follows directly from the fact that the reaction stoichiometry becomes raised
to the power of the coefficient inside the logarithm.
,
follows directly from the fact that the reaction stoichiometry becomes raised
to the power of the coefficient inside the logarithm.