| |
Solvation of dipolar molecules
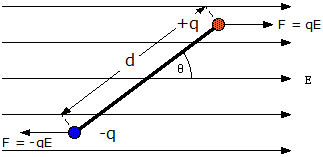
Charged species (ions) are discussed in fundamental courses in Chemistry since these are needed to understand acid/base chemistry, electrochemistry and so on. However, molecules have a more complicated charge distribution in general. Even neutral molecules have a dipole. In the general case, we may consider other moments as well, such as quadrupole, octapole etc. This terms are part of the multipole expansion. The dipolar term is by far the largest and therefore the most important for our discussion. We will consider how dipoles are aligned in an electric field and how dipoles interact in order to help construct a fundamental theory of solvation of dipolar molecules. What makes this subject particularly important is that many solvents consist of dipolar molecules. These are called polar solvents. In fact, the entire topic is so important that one of the classic books in Chemistry is Peter Debye's 1929 book called "Polar Molecules". It is still worth reading today since it explains many aspects of this important area in a short and well-written book.
Dielectric polarization
When a sample containing dipoles is subjected to an electric field, the dipoles will tend to align in the direction of the field. This property permits us to consider the effects of dipole-dipole interactions on the tendency to align. Dipoles can shield other dipoles, just as charges can shield other charges. The shielding by dipoles is the phenomenon that gives rise to the dielectric function. When speaking loosely the dielectric function is called a dielectric constant. For example, we often will say that the dielectric constant of water is 82. But, this value is temperature and frequency dependent. Therefore, it is a function of both omega and T.
Molecular polarizability
| |
|
|
|